Sleep trials
We have 2 sleep units, each with 4 bedrooms –
- 4 rooms with en-suite toilet and shower
- 4 rooms with adjacent toilet facilities
Features include
- high-level sound-proofing, with acoustic boards, acoustic tiles & 10 cm thick fibre insulation
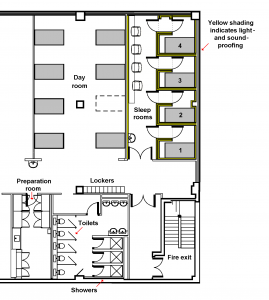
- no windows
- entrance lobby maintains sound- & light-proofing
- remote-controlled air conditioning & ventilation
- electrical trunking with separate channels for mains and low-voltage cables
- shielding of all mains cables, & wooden beds, to prevent electromagnetic interference
- infrared light & video
- controlled light for <5 lux waking environment
- microphone for sound monitoring
- external port for remote intravenous blood sampling
- Embla N7000 & MDrive polysomnography (PSG) systems with EEG, EOG, ECG & EMG electrodes & leads; oximeter, respiratory effort, body position, nasal cannula, thermistor & snoring sensors
We’ve 6 acquisition PCs, each on UPS, that store data on mirrored RAID drives. We copy data across the network to our file server, which we back up scrupulously.
We validate PSG equipment in accord with 21 CFR part 11. We’ve trained staff, and links with academic units experienced in sleep research.
We work according to AASM-recommended technical standards, to produce data acceptable for peer-reviewed publication and regulatory approval.
We can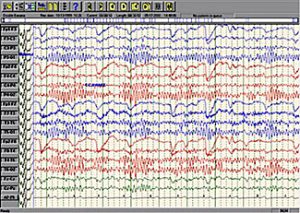
- help with trial design
- analyse data onsite for interim results and final reports
- send data via FTP in EDF format
In addition to the sleep unit, we have
- extensive experience in phase 1 trials of novel CNS drugs, including first-in-man
- experience of sleep deprivation and circadian rhythm trials
- extensive experience of CNS procedures
- EEG
- psychomotor tests
- coordination tests
- cognitive function
- MIA(IMP) to manufacture sterile & non-sterile products
- MHRA Phase 1 Accreditation
