 We provide efficient, high-quality data management and statistical support for early phase clinical trials conducted at HMR or external sites. We follow CDISC (Clinical Data Interchange Standards Consortium) standards (including CDASH and SDTM) to maximise efficiency and to ensure that our data comply with regulatory requirements. We offer a flexible, bespoke service, using either our own or our customers ‘ templates and standards.
We provide efficient, high-quality data management and statistical support for early phase clinical trials conducted at HMR or external sites. We follow CDISC (Clinical Data Interchange Standards Consortium) standards (including CDASH and SDTM) to maximise efficiency and to ensure that our data comply with regulatory requirements. We offer a flexible, bespoke service, using either our own or our customers ‘ templates and standards.
We pride ourselves in providing high-quality data on time, every time.
CRFs and databases – we offer electronic and paper-based data management. Both systems allow us to deploy databases rapidly and to respond quickly to protocol amendments. All our systems are 21 CFR Part 11 compliant and validated for use by HMR.
- Electronic data management (eDM) – we use Medrio e-clinical, coupled with HMR templates, to design and build the eCRF and database. HMR staff enter the data on-site. Customers benefit from rapid, remote access to their data in Medrio e-clinical.
- Paper-based data management – we design a paper CRF using the HMR template, and build the database in ClinPlus, a SAS-based data management system from DZS Software Solutions. Our experienced data entry operators do double data entry.
Data checks – we write a comprehensive specification for data validation, for each database. We program each check using standard macros for efficiency, and test them using correct and incorrect dummy data.
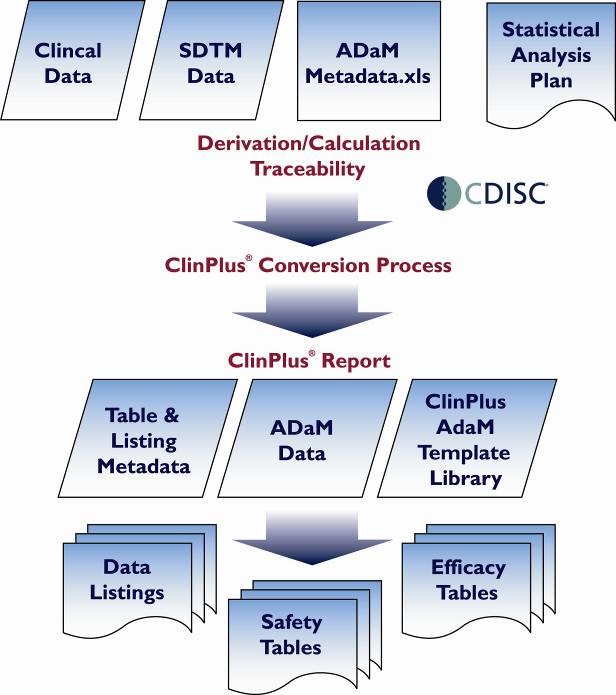
Query management – we manage programmed and manual queries via the data management software. We actively manage the query process to ensure rapid resolution.
Medical coding – we code medical history, adverse events and medication, using MedDRA and WHO DDE.
Data reconciliation – before database lock, we reconcile serious adverse events in our database with pharmacovigilance databases, and we reconcile other external electronic data.
Database lock – we follow comprehensive quality control procedures before locking each database, to ensure that we deliver high quality data, every time.
Data transfer – we transfer interim and final data to customers in SDTM, or in their requested format.
