Since 1993, we’ve done more than 800 phase 1 trials, over 200 of which involved compounds with CNS (Central Nervous System) activity. Many of those trials were first-in-human. We’ve experience of a wide range of procedures to assess novel CNS compounds, including
- sleep and polysomnography
- actigraphy
- scopolamine model of dementia
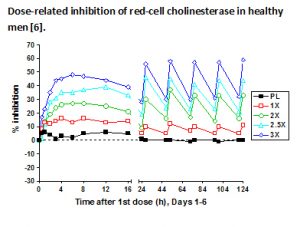
- erythrocyte cholinesterase activity
- pain models
- EEG, with interpretation by consultant neurologist
- PET, with Invicro
- SPECT, with University College Hospital, London
- fMRI, with Invicro or Institute of Psychiatry, Kings College Hospital
Types of molecules that we’ve studied include
- dopamine receptor antagonists
- anticonvulsants
- antipsychotics
- antidepressants
- GABA inverse agonists
- cholinesterase inhibitors

Collaboration with other specialist providers
We collaborate with specialists in products and services in CNS experimental medicine. For example, we’ve worked with cognitive assessment platforms such as CANTAB and Cogstate, which offer validated and reliable assessment of CNS functions
HMR publications
- Quelch DR, Mick I, McGonigle J, van den Berg F, Boyce M, et al. Nalmefene reduces reward anticipation in alcohol dependence – an experimental functional magnetic resonance imaging study. Biol Psychiatry 2017; 81: 941–948
- Antonova E, Parslow D, Brammer M, et al. Scopolamine disrupts hippocampal activity during allocentric spatial memory in humans: an fMRI study using a virtual reality analogue of the Morris Water Maze. J Psychopharmacol 2011; 25: 1256–1265
- Addy C, Li S, Warrington S et al. Safety, tolerability, pharmacokinetics and pharmacodynamic properties of taranabant, a novel selective cannabinoid-1 receptor inverse agonist, for the treatment of obesity: results from a double-blind, placebo-controlled, single oral dose study in healthy volunteers. J Clin Pharm 2008; 48: 418–427
- Norris V, Baisley KJ, Calder N, van Troostenburg AR, Warrington SJ. Assessment of the Accusway Plus system in measuring the effect of lorazepam on body sway in healthy volunteers.
Int J Pharm Med 2005; 19: 233–238 - Clarke A, Johnson ES, Mallard N, Corn TH, Johnston A, Boyce M, Warrington S, MacMahon DG. A new low-dose formulation of selegiline: clinical efficacy, patient preference and selectivity for MAO-B inhibition. J Neural Transmission 2003; 110: 1257–1271
- Johnson N, Cattoni M, Warrington S, Boyce M. Tolerability and pharmacodynamic effects of repeated doses of ganstigmine, a new cholinesterase inhibitor, in healthy men. Br J Clinical Pharmacol 2000; 49: 492P–493P